

When you hear the word vacuum, you probably think of that annoyingly loud machine we use to clean our houses, a.k.a the vacuum cleaner (or maybe even the little robot that automatically cleans your house for you). But in the world of science, a vacuum refers to a space that has no matter.
Think about it this way: take a second to imagine an empty container. What's inside of it? Trick question, but we think you got it right—the answer isn't "nothing," it's "air" of course! Because on Earth, our atmosphere surrounds us, the concept a vacuum is a bit, well, weird. But what happens if we somehow suck all the air out of that container? If you said we'd have created a vacuum, you'd be right!
In reality, however, it's impossible to create a perfect vacuum. Instead, scientists can approximate a vacuum by removing as much air as possible from a container, thereby creating a huge difference between the air pressure inside the container and the atmospheric pressure outside the container. Like in a vacuum cleaner, this difference in pressure creates a situation where air molecules try to rush into the vacuum to fill the empty space. This creates the "sucking" force we associate with vacuum cleaners, straws, and more!
In this demonstration, we're going to create a simple vacuum by heating up the air in a closed-off glass. When the air cools, the pressure inside the glass will be so low that air from outside the glass will try to rush back inside. But instead of air... well, we'll see what happens shortly!

Add enough water to just cover the bottom of the bowl.

(Optional) If you like, you can add some food coloring to the water now––two or three drops should do it!

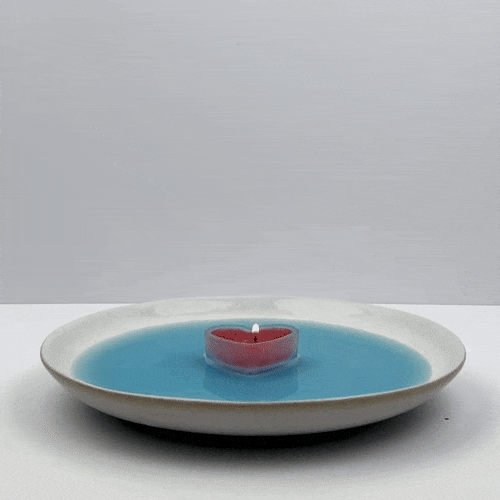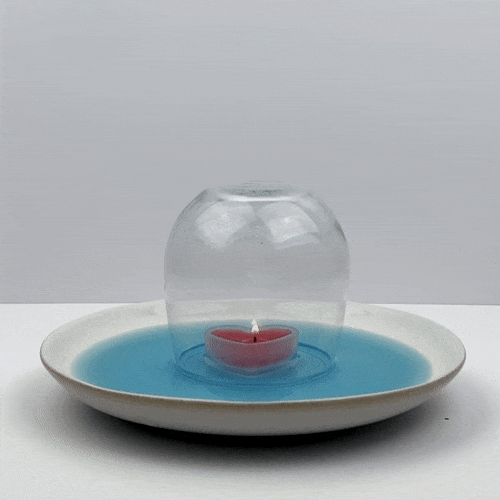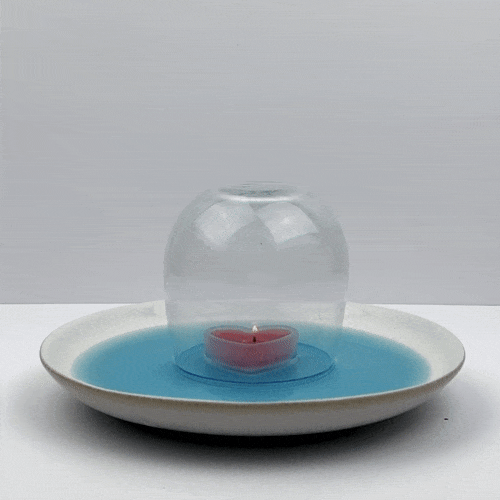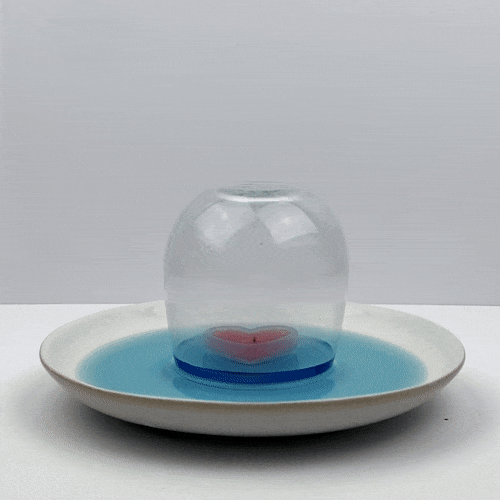
Do not go any further without adult permission and supervision. Now, place the candle in the center of the plate and light it with the match.

Place the glass upside down over the candle. Make sure that the water forms a seal on the glass, so that no air can enter the glass.

Observe your candle-powered vacuum in action. After the water level has stopped changing, what can you say about the air pressure in the top of the glass? Should it be higher, lower, or almost the same as the pressure of our atmosphere?
You must be logged in to comment.