

Molecules are groups of two or more atoms, held together with chemical bonds. In other words, they are the smallest unit of a chemical compound.
Molecules come in all shapes and sizes, ranging from simple diatomic (two-atom) molecules like oxygen (O2) to complex biomolecules like DNA, which can contain many thousands of atoms. So, while there are only 118 different elements on the periodic table, there is an almost endless number of ways to combine atoms into different molecules.
Chemical bonds are formed when atoms share their electrons in different ways. The way that a molecule's atoms are bonded together determines its properties and behavior, such as its shape, size, and chemical reactivity.
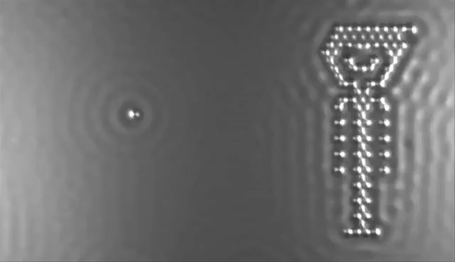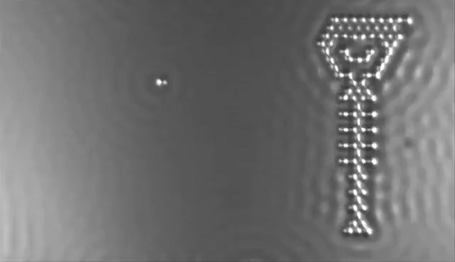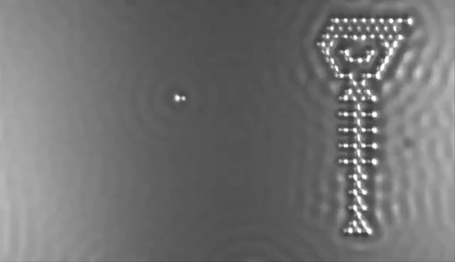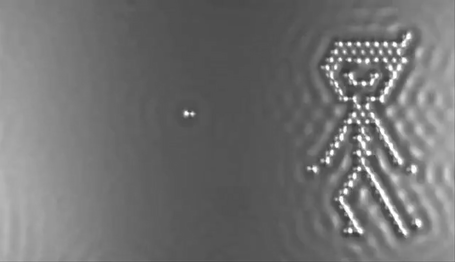 In 2013 the computer manufacturer IBM released a stop-motion movie onto YouTube entitled, A Boy and His Atom. The movie was made by taking pictures with a scanning electron microscope, where individual molecules of carbon monoxide (CO) are magnified over 100 million times in size. The molecules were then moved around to create the images of a boy dancing and playing with an atom.
In 2013 the computer manufacturer IBM released a stop-motion movie onto YouTube entitled, A Boy and His Atom. The movie was made by taking pictures with a scanning electron microscope, where individual molecules of carbon monoxide (CO) are magnified over 100 million times in size. The molecules were then moved around to create the images of a boy dancing and playing with an atom.
Adenosine triphosphate (ATP) is a molecule found in all living things, from tiny bacteria and plants to giant whales. ATP is often called the "energy currency" of cells because it's used to store energy for cellular processes, like making proteins or moving muscles.
ATP is made up of three parts: a sugar called ribose, a nitrogen (N) molecule called adenine, and three groups of atoms containing phosphorus (P). When cells need energy, they break a bond in an ATP molecule, which turns it into ADP (adenosine diphosphate) and releases energy.
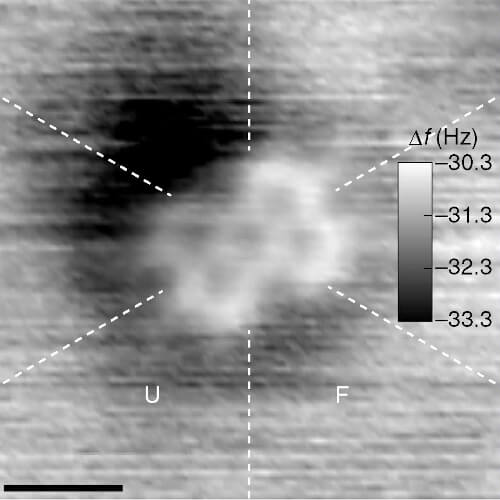
Titin is a protein found in muscles, and the largest molecule found in nature. Like all other proteins, titin is a chain of many smaller molecules called amino acids. Its full chemical name has 189,819 letters, which come from combination of chemical symbols, numbers, and dashes, representing the structure and order of the amino acids in the protein.
Buckminsterfullerene is a molecule made up of 60 carbon atoms arranged in a spherical, or ball, shape. It's named after the inventor Buckminster Fuller because it kind of looks like some of his creations, called geodisk domes.
Isomers are molecules that have the same chemical formula, or exact same amount and types of atoms, but with different shapes. Think of it like building with Legos. Even if you have two kits with the same exact Lego pieces, you can still use them to build different things, depending on how you arrange the pieces. Even though they have the same chemical formulas, isomers can have chemical properties that are quite different from one another.
PG5 is a synthetic, or lab-made, molecule, containing about 200 million atoms. It was created by chemists at the Federal Institute of Technology in Zürich. Before its creation, polystyrene, also known as styrofoam, was the largest known molecule, containing about 40 million atoms.